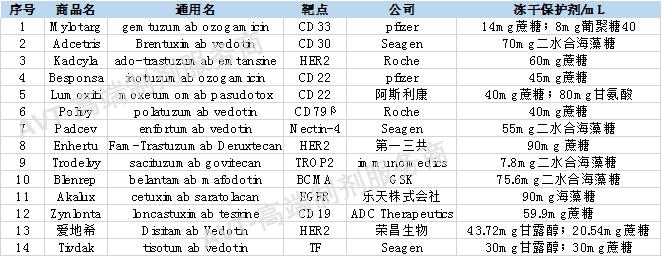
As of June 2022, a total of 14 antibody-drug conjugates (ADCs) have been approved worldwide.
Generally, the components of any ADC include:
① ADC (active ingredient); ② lyoprotectant & excipient (sugar or polyol); ③ pH buffer (such as histidine, Tris); ④ surfactant (such as Tween 80, Tween 20).
All of the 14 listed ADCs are lyophilized powder formulations. The basic information and the use of lyophilized protectants in the drug product are shown in the table below:
The active ingredients of biological drugs are biological macromolecules (such as antibody proteins, enzymes, mRNAs, etc.), which are easy to degrade and denature. The conditions required for maintaining the physical and chemical properties and activity of biological drugs are much higher than those of small-molecule chemical drugs. Most of liquid biological drugs are unstable at room temperature and may undergo various physicochemical reactions, including hydrolysis, cross-linking, aggregation, deamidation, and disulfide, affecting their biological activities.
The main advantages of changing liquid dosage forms into lyophilized powder dosage forms by freeze-drying technology are as follows:
① The long-term stability of the drug product is improved;
② The physical and chemical properties and biological activity of the drug product are effectively retained;
③ The weight of the finished product is reduced by more than 90%, lowering the requirements for storage and transportation and improving accessibility.
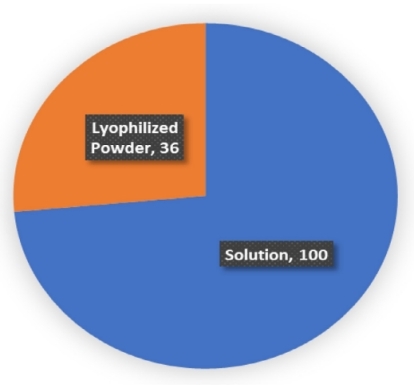
Lyophilization technology has been widely used in biological drugs. As of February 2021, more than 125 antibody drugs have been approved by the FDA since the first monoclonal antibody (Orthclone) was marketed in 1986. Of all 136 drug products, 36 were lyophilized powder formulations, accounting for over 25%.
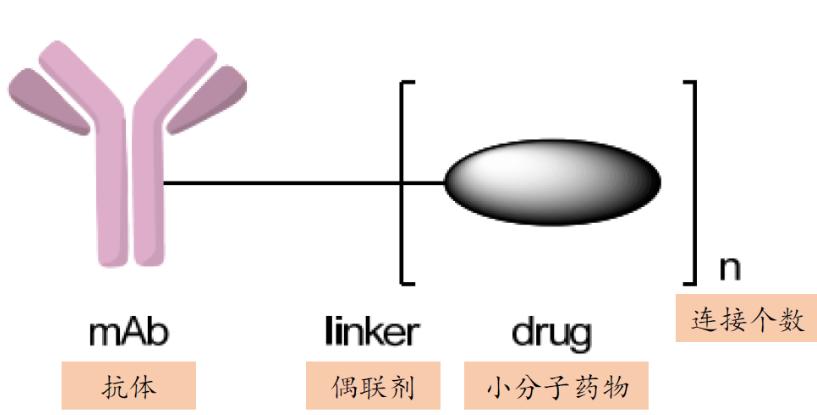
ADCs are composed of antibody + linker (coupling agent) + small-molecule drugs, and their structures are more complex than traditional antibody drugs, requiring higher long-term stability of the drug product. In view of this, lyophilized dosage forms have more advantages over liquid dosage forms.
There are various stresses in freeze-drying process, mainly including low-temperature stress, freezing stress, drying stress, etc. Biological drugs have the risk of inactivation of biological macromolecules such as proteins during freezing or lyophilization, so protectants need be added to improve stability.
Lyophilized protectants mainly play the following roles:
① Providing protection for the drug product throughout the lyophilization process (divided into freezing process and drying process);
② Inhibiting the denaturation/degradation/inactivation of biomacromolecules in the finished products during the storage period.
Carbohydrates are the most commonly used lyoprotectants and excipients in lyophilized protein drugs, and their structures with many hydroxyl groups may provide stabilization by replacing hydrogen bonds between proteins and water. In addition, most carbohydrates do not crystallize under normal lyophilization conditions, resulting that lyophilized protein products can be reconstituted more rapidly.
It should be noted that reducing sugars (e.g., lactose, maltose, etc.) are not typically used for freezing and drying because they denature and inactivate the protein by reacting with the protein through a Maillard reaction.
For lyophilized biological drugs, sucrose and trehalose are the most commonly used carbohydrate protectants. Sucrose has been widely used in protein drugs, and trehalose for sale is considered to be a superior lyoprotectant than sucrose because it has a higher glass transition temperature. Trehalose has other advantages, such as low hygroscopicity and high viscosity. The high viscosity of sucrose and trehalose also reduces the rate of protein denaturation during drying at temperatures above the glass transition temperature, thereby increasing the freeze-drying efficiency.
Of the 14 listed ADCs, 9 used sucrose and 5 used trehalose. Of the 36 marketed lyophilized powder antibody drugs, sucrose was used in 23 products and trehalose (one of which was trehalose + sorbitol) in 11 ones as a lyophilized protectant.
In addition, both sucrose and mannitol are used in two ADCs. Polyols can stabilize proteins through their hydroxyl groups and act as cryoprotectants and lyoprotectants. However, polyols are far less widely used in lyophilized biological drugs than sugars because they have disadvantages such as weaker binding ability to proteins, easy cake collapse due to freezing in amorphous form, and possible vial rupture during lyophilization due to recrystallization.
Based on the above analysis, sucrose and trehalose are preferred as lyophilized protectants for ADCs, antibody drugs and other lyophilized biological products. If the lyophilization effect of carbohydrate protectant alone is ineffective, the combination of carbohydrate and polyol can be tried.
When screening lyophilized formulations and trying different sugars and polyols, there are many combinations and types. Generally, 10% trehalose, 9% sucrose, and 5% sorbitol can be taken as the preliminary basic formula, and if they are to be combined, the amounts of the two are halved. After standing at 4°C, 25°C and 40°C for 14 days, the clarity, BCA concentration, purity by SEC-HPLC, thermal stability by DSC and acid-base peak content by CIEF were observed macroscopically.
AVT supplies parenteral grade trehalose, sucrose for sale, TRIS/TRIS-HCl buffer, HEPES and other bioprotectants and buffer solutions on a permanent basis. Our parenteral-grade products with ultra-low endotoxin are manufactured in accordance with the current GMP, and have registered in both China and America, meeting the pharmacopoeia standards of various countries. With our products, we contribute to your manufacturing and registration in China and/or other countries of mRNA-LNP, liposomes, single and double antibodies, ADCs and other biological drugs!