Buffer salts (for Biological) belong to biological buffer systems, which contain dozens of varieties like buffered electrolyte salts,Tris and HEPES.
Buffered electrolyte salts are a type of buffer salt that can help maintain the ionic balance of biological systems.The advantages of buffered electrolyte salts including high purity, low impurities, production in accordance with GMP guideline, stable supply and high cost-effective.
Tris is a buffered salt widely used in the field of biomedicine. Buffered Salt is a combination mineral supplement that may be helpful in reducing tiredness. AVT has newly launched tromethamine (TRIS), which is in the process of NMPA and US FDA registration.
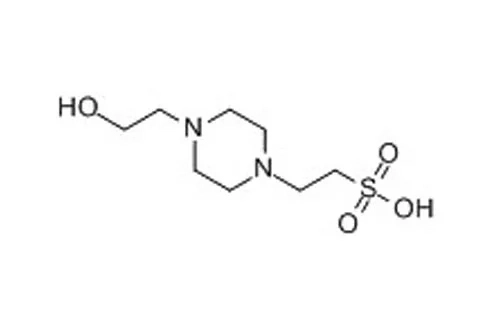
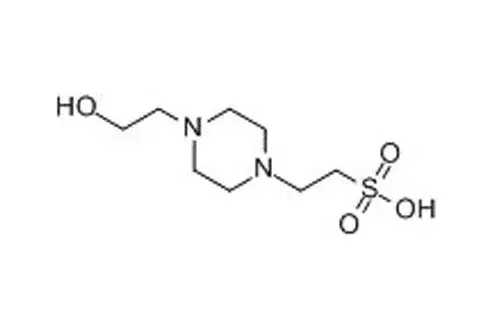
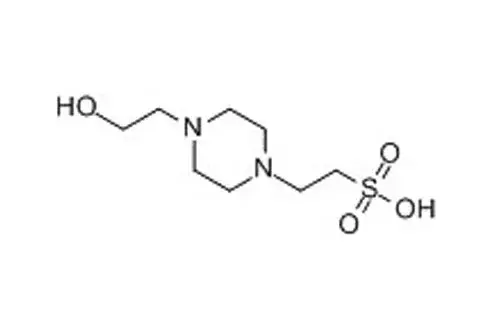
HEPES, short for 2-(4-(2-hydroxyethyl)-1-piperazinyl)-ethanesulfonic acid, is another commonly used buffer salt known for its ability to maintain pH at physiological levels. Both Tris and HEPES are widely used in research and industries such as biotechnology and pharmaceuticals.
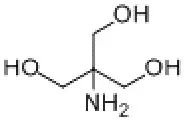
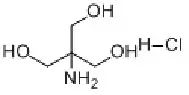
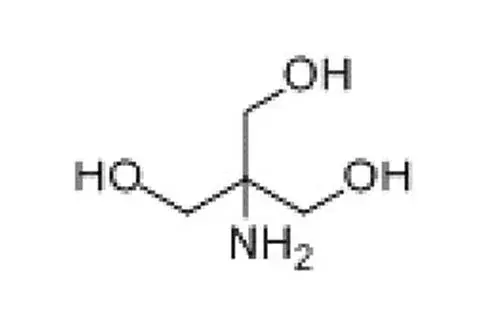
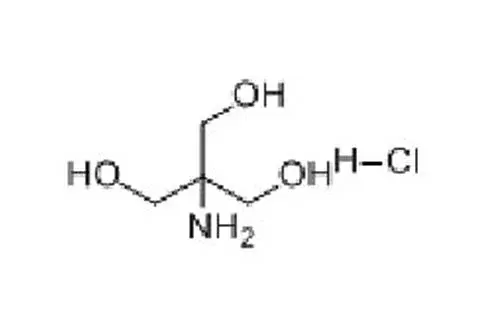
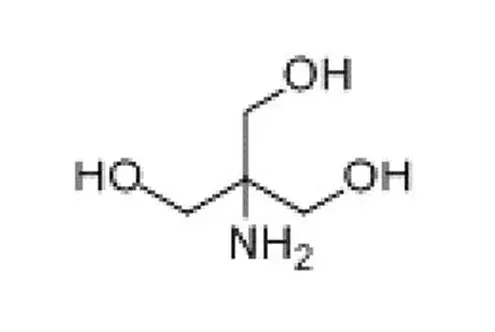
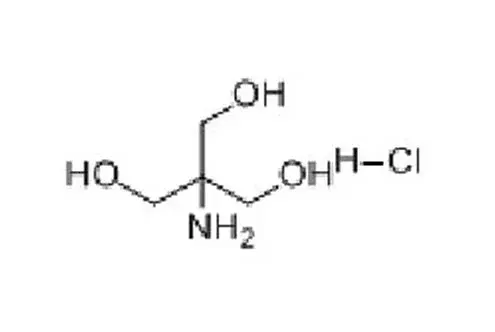
Tromethamine (Tris) is a buffer salt widely used in the field of biomedicine. Chemical formula is C4H11NO3. It is white crystalline particles, commonly used in protein or nucleic acid buffers. The effective range is usually between pH 7.0-9.2.
Tris is used for the growth of protein crystals under different pH conditions. Tris is also one of the main components of protein electrophoresis buffer. It forms a buffer system with glycine in the electrophoresis buffer to stabilize the pH during electrophoresis.
In addition, Tris is also an intermediate in the preparation of surfactants, vulcanization accelerators and some drugs. Titration standard substance is also its usage.
1. The basicity of Tris base is strong, so we can use only this buffer system to prepare buffers with a wide range of pH ranging from acidic to basic. The application range is wide.
2. Tris buffer has little interference with biochemical processes and does not precipitate with calcium, magnesium ions and heavy metal ions.
3. Tris is highly soluble in water and is inert to many enzyme reactions.
4. Tris has high buffering capacity, especially between pH 7.5-9.0.
5. Tris buffer is widely used and plays an important role in biochemistry, molecular biology, in vitro diagnosis, cosmetics, coatings and other fields.
If you have any questions, please don't hesitate to contact us. We're always here to help with Pharmaceutical Excipients for your business. We can provide products ranging from grams to tons. AVT is able to produce and even register the product in US FDA according to your special needs.