Lipid nanoparticles (LNPs) have emerged across the pharmaceutical industry as promising vehicles to deliver a variety of therapeutics. LNPs hold great promise in genetic medicine where gene editing, vaccine development, immuno-oncology, and other genetic therapies rely on the ability to efficiently deliver nucleic acids into cells. LNPs have advantages over other gene and vaccine delivery systems because they are easier to manufacture, are less immunogenic, can carry larger payloads, and can be designed for multiple dosages. The successful use of LNPs as a delivery vector for the COVID-19 mRNA vaccines will likely broaden the horizons for research in mRNA vaccines.
| classification | Product | ||||||
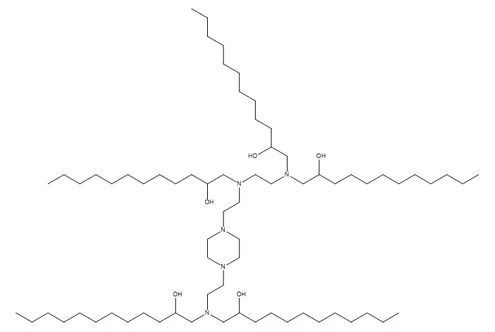
| Cationic Lipid | Dlin-MC3-DMA | SM-102 | ALC-0315 | DC-CHOL | DOTAP | DOTMA | DODMA |
| Polyethylene Glycol(PEG) Lipids | DMG-PEG2000 | ALC-0159 | DSG-PEG2000 | ||||
| Structural | DSPC | DOPC | |||||
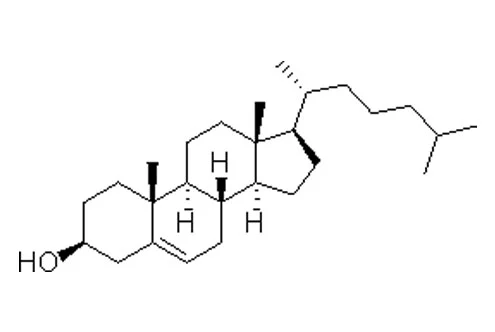
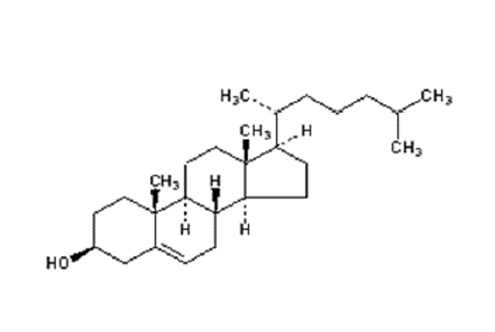
| Helper lipid | CHO(lanolin origin) | CHO(plant origin) | DOPE | ||||
| Other excipients | Sucrose | Trehalose Dihydrate | Tromethamine | Trometamol hydrochloride | |||


If you have any questions, please don't hesitate to contact us. We're always here to help with Pharmaceutical Excipients for your business. We can provide products ranging from grams to tons. AVT is able to produce and even register the product in US FDA according to your special needs.