In the previous two issues, we shared relevant studies on the use of D(+)-trehalose dihydrate for cryopreservation of human-derived oligodendrocyte precursor cells (OPCs) and adipose-derived stem cells (ADSCs). Those studies found that adding a certain amount of D(+)-trehalose dihydrate to the cryopreservation medium or using the composite cryoprotectant of D(+)-trehalose dihydrate + glycerol could improve the recovery rate of stem cells after long-term cryopreservation, thus contributing to the subsequent clinical application of stem cells for disease treatment or wound repair.
In this issue, we take cryopreservation of mouse testicular germ cells as an example to explore the application prospects of D(+)-trehalose dihydrate in the field of assisted reproduction.
Effects of Different Cryoprotectants on Cryopreservation of Testicular Germ Cells in Immature Mice
In recent years, with the rapid development of assisted reproductive technology, it has become possible for more and more infertile men to give birth to offspring. In the process, long-term cryopreservation of reproduction-associated cells has become one of the key technical difficulties affecting the success rate.
For example, for prepubertal male patients with cancer, treatment with gonadal toxicity may lead to permanent loss of fertility, and long-term cryopreservation of testicular tissue or cells at low temperatures is an important means of preserving their fertility.
However, for patients with systemic tumors, autologous transplantation of cryopreserved testicular tissue after thawing poses a risk of reintroduction of cancer cells. Considering that cancer cells can be isolated from testicular cell suspension and spermatogonial stem cells (SSCs) can be cultured in vitro to induce differentiation into round spermatids, giving birth to offspring can be achieved by round spermatid injection.
Therefore, for prepubertal male patients with cancer, long-term cryopreservation of testicular germ cells is one of the key ways to preserve male fertility and has potential clinical application value.
Zhang Xiaolong from Henan Provincial People's Hospital and other researchers used two-week-old mice to simulate the development state of human immature testicular tissue before puberty, and adopted different cryopreservation protocols to cryopreserve mouse testicular germ cells to explore the best cryopreservation method.
In the study, the cryopreservation media were divided into the following 5 groups according to the different components:
Group | Cryopreservation Medium Composition |
A | DMEM/F12 medium containing 10% DMSO + 10% FBS |
B | DMEM/F12 medium containing 10% DMSO + 10% FBS + 0.1 mol/L sucrose |
C | DMEM/F12 medium containing 10% DMSO + 10% FBS + 0.1 mol/L D(+)-trehalose dihydrate |
D | DMEM/F12 medium containing 15% DMSO + 10% FBS + 0.1 mol/L sucrose + 0.1 mol/L D(+)-trehalose dihydrate |
E | DMEM/F12 medium containing 10% DMSO + 10% FBS + 0.1 mol/L sucrose + 0.1 mol/L D(+)-trehalose dihydrate |
Control | Testicular germ cells dissociated from fresh mouse testicular tissue (without cryopreservation medium) |
Mouse testicular germ cell suspension was prepared by two-step enzymatic hydrolysis, dispensed, and transferred to cryogenic vials after adding each of the five different cryoprotectants respectively. The cryogenic vials were subject to programmed freezing, taken out from the liquid nitrogen tank after 2 months, and recovered to detect the cell survival rate, apoptosis rate, and mitochondrial membrane potential.
① Cell survival rate and recovery rate before and after cryopreservation
Compared with that before cryopreservation, the cell survival rate after cryopreservation decreased in all the five test groups. Among them, the cell survival rate and recovery rate in group E were significantly higher than those in the other four test groups.
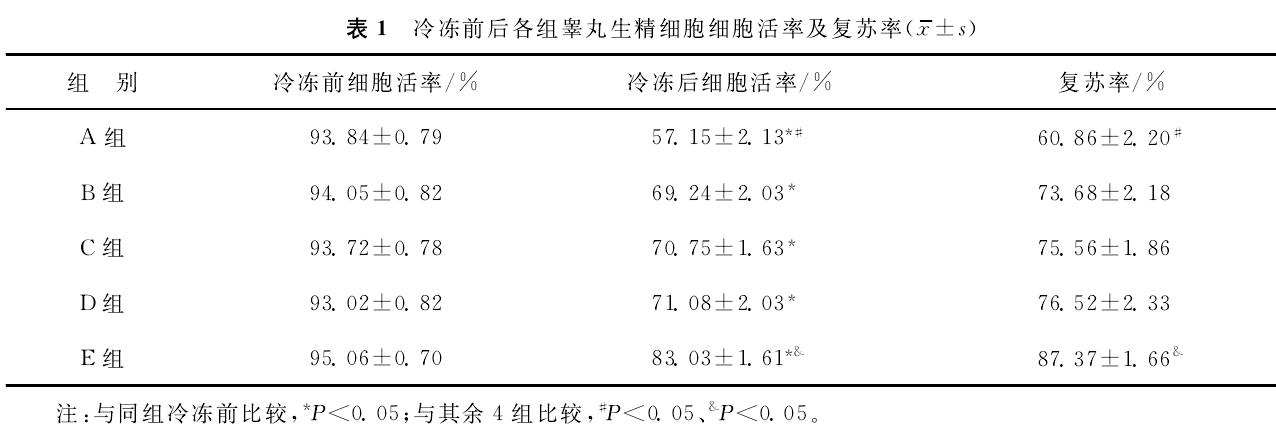
② Cell recovery after cryopreservation
In each group, the recovery rate of testicular germ cells after cryopreservation was quantitatively evaluated based on the number of viable cells actually recovered from 4 mg of dissociated and cryopreserved testicular tissue after thawing. The results showed that the number of cells recovered in group E was significantly higher than that in the other four test groups.
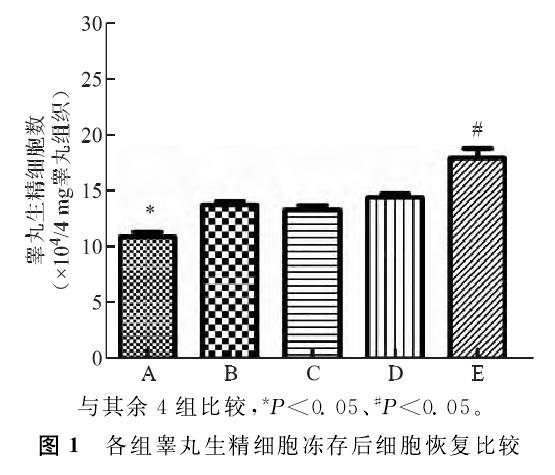
③ Apoptosis rate
The apoptosis rate was detected by flow cytometry (FC) in each group. The results showed that the apoptosis rate was significantly increased in each test group compared with the control group. In the five test groups, the apoptosis rate in group E was significantly lower than that in the other four test groups.
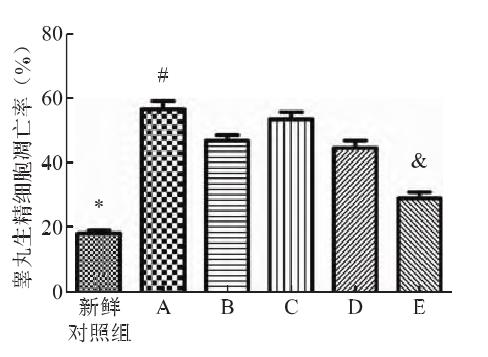
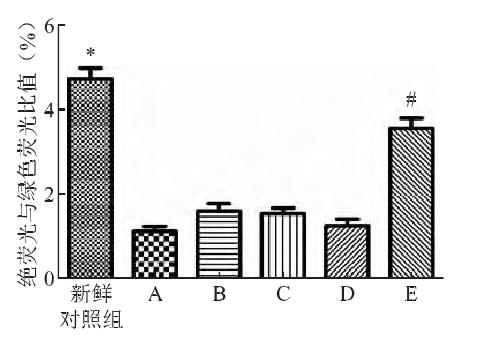
④ Mitochondrial membrane potential
Compared with the control group, the mitochondrial membrane potential of the cryopreserved cells decreased significantly after thawing in the five test groups, with the smallest decrease in group E, which was significantly lower than that in the other four test groups.
Cryoprotectants can be classified as permeable and impermeable. The most commonly used impermeable cryoprotectants are sucrose and D(+)-trehalose dihydrate. A previous study has confirmed that adding sucrose or D(+)-trehalose dihydrate to cryoprotectants could significantly increase the recovery rate of testicular germ cells in rhesus monkeys or mice.
The results of the above study showed that adding 0.1 mol/L sucrose or 0.1 mol/L D(+)-trehalose dihydrate to the cryoprotectant had a certain protective effect on cell cryopreservation, while adding 0.1 mol/L sucrose and 0.1 mol/L D(+)-trehalose dihydrate at the same time significantly increased the recovery rate of cryopreserved mouse testicular germ cells after thawing, decreased the apoptosis rate, and reduced the damage to the mitochondrial membrane potential caused by cryopreservation.
However, the cytotoxicity caused by the higher DMSO concentration (15%) partially offset the cryoprotective effect of sucrose and D(+)-trehalose dihydrate.