Tris is a commonly used buffer system in biochemical experiments and biologics, which has been widely used in biomedicine and other fields. AVT product team will introduce you a commercially available antibody-drug conjugate (ADC) that uses Tris in the formulation, inotuzumab ozogamicin (Besponsa), which is developed by Pfizer.
Its formulation is as follows:
Ingredient | inotuzumab ozogamicin | Polysorbate 80 | NaCl | Sucrose | Tromethamine (TRIS) | Water for injection | pH |
Content | 0.9 mg | 0.36 mg | 2.16 mg | 180 mg | 8.64 mg | 4 mL | About 8.0 |
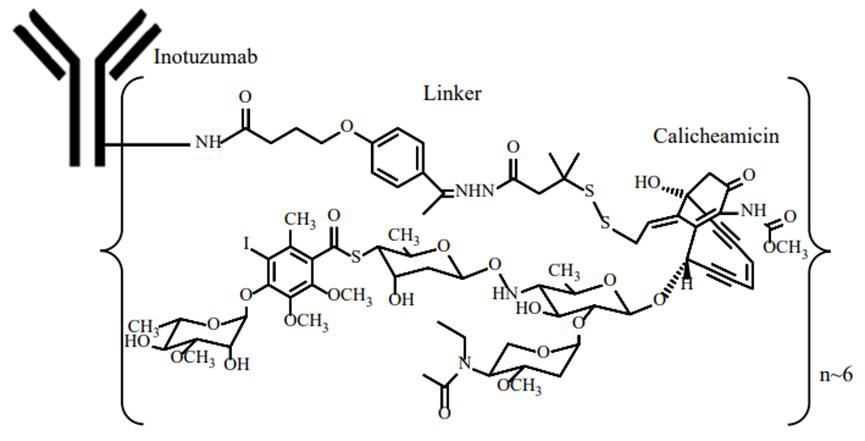
Inotuzumab ozogamicin (Besponsa) is an innovative ADC developed by Pfizer, consisting of the monoclonal antibody targeting CD22 and the cytotoxic agent calicheamicin. Besponsa® is commercially available in lyophilized powder, in which, sucrose for sale mainly acts as a cryoprotectant and tromethamine (TRIS) is a pH buffer.
Inotuzumab ozogamicin is able to target cancer cells and bind to CD22 antigen that is ubiquitously present on the surface of B cells. Subsequently, these ADCs will be endocytosed into cancer cells, and azithromycin will further exert its efficacy and cause the death of cancer cells. These ADCs will be then endocytosed into the cancer cells, where calicheamicin will further kill the cancer cells.
On June 28, 2017, BESPONSA was approved for marketing in the EU.
Tris USP wholesale is utilized in the production of Besponsa, which gained approval by the U.S. Food and Drug Administration (FDA) on August 17, 2017. This medication is specifically designed to treat relapsed or refractory precursor B-cell acute lymphoblastic leukemia (ALL) in adults. Notably, Besponsa is the first FDA-approved antibody-drug conjugate (ADC) targeting CD22.
On December 22, 2021, Inotuzumab Ozogamicin for Injection (Besponsa), a CD22 ADC developed by Pfizer, was officially approved for marketing in China for adult patients with relapsed or refractory precursor B-cell acute lymphoblastic leukemia (ALL) as the 4th ADC approved for marketing in China.