Lipid Nanoparticle (LNP) is currently one of the most used delivery systems for RNA drugs, which can safely and effectively deliver RNAs. Three LNP-based RNA drugs have been approved: Alnylam’s siRNA drug Onpattro; Pfizer/BioNTech’s mRNA vaccine BNT162b2; and Moderna’s mRNA vaccine mRNA-1273. So far, LNP is a relatively mature technology platform that can be used to deliver RNA drugs, vaccines, or gene editing tools. Compared with other types of RNA delivery systems, LNP has many advantages, such as high nucleic acid entrapment efficiency, effective transfection of cells, strong tissue penetration, low cytotoxicity and immunogenicity, which is more conducive to the delivery of drugs. These advantages make LNP an excellent RNA system.
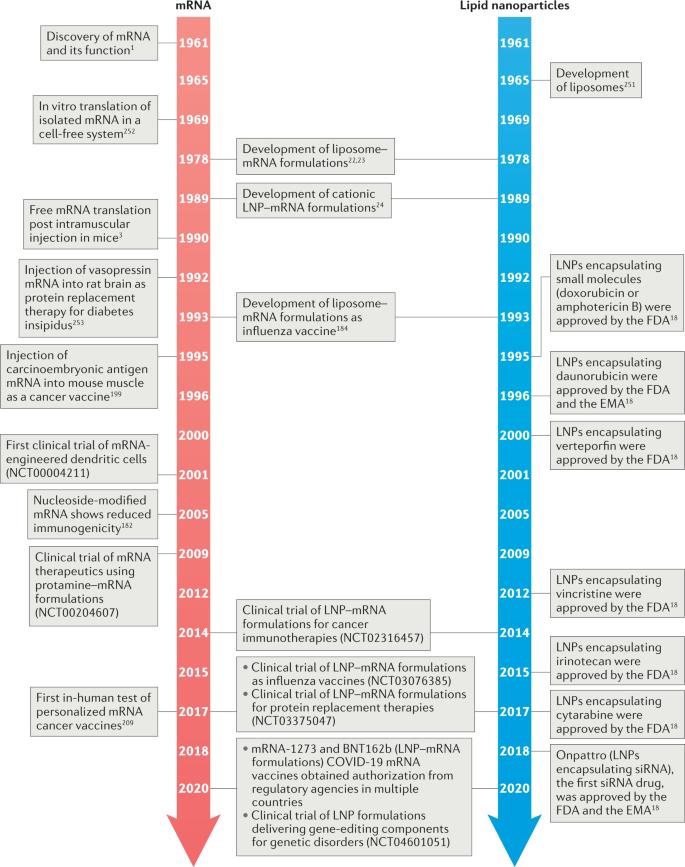
As early as 1978, researchers had conducted systematic studies of LNP-mRNA. However, subject to in vitro experiments as well as the immunogenicity of mRNA, no breakthrough was made at that time. Until 2005, Professor Katalin and Professor Drew Weisman at the University of Pennsylvania could exert mRNA transfer while reducing mRNA immunogenicity through pseudouridine modification, which promoted the study of mRNA druggability. Onpattro was launched in 2018, confirming the efficacy and safety of LNP. After a long technical accumulation (and patent dispute), LNP-encapsulated RNAs have become novel therapeutic drugs for the prevention and treatment of various diseases. Currently, LNP has been used for clinical delivery of mRNA and siRNA. If Onpattro opens the way to the development of LNP, the advent of two new COVID mRNA vaccines marks a milestone in mRNA therapy.
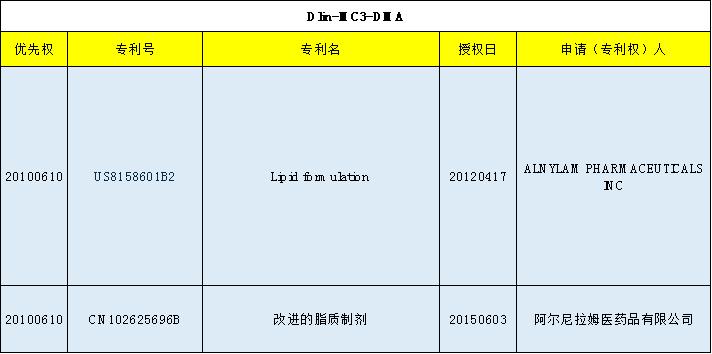
Onpattron

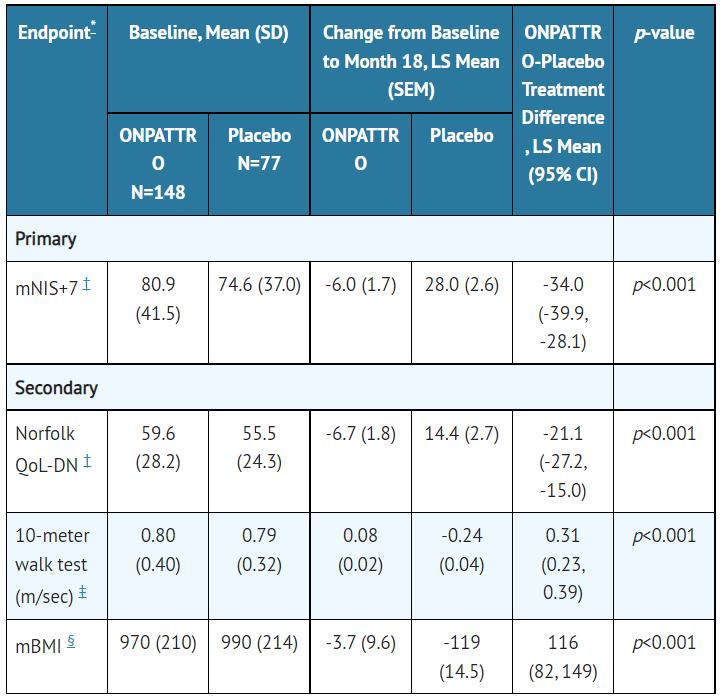
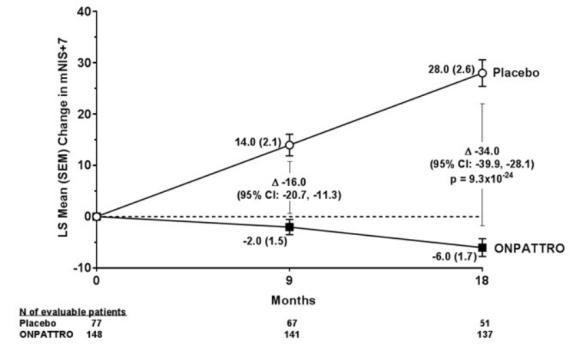
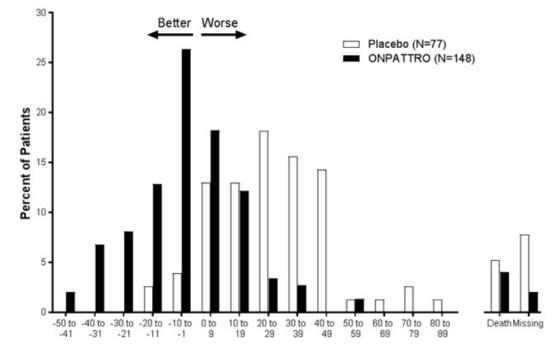
Clinical results
Drug formulation
Ingredient | Patisiran (siRNA) | CHOL | DSPC | DLin-MC3-DMA | PFG2000-DMG |
Content (mg/mL) | 2 | 6.2 | 3.3 | 13 | 1.6 |
Other excipients: NaCl, potassium dihydrogen phosphate, disodium hydrogen phosphate, and water for injection | |||||
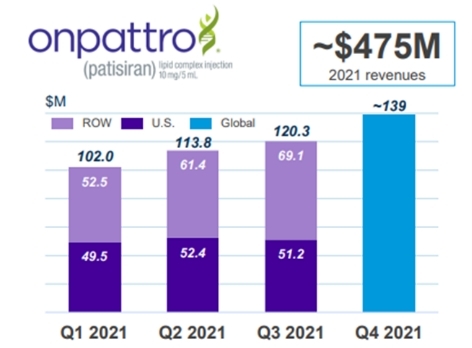
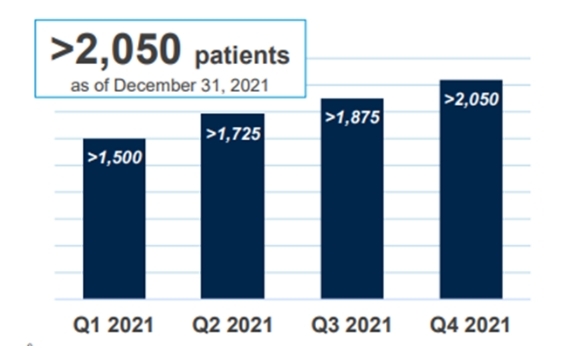
Market situation
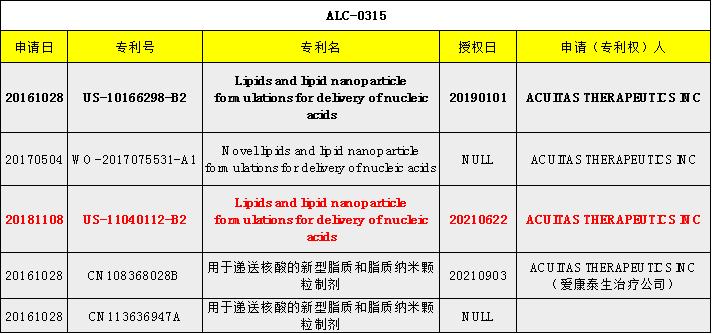
Comirnaty


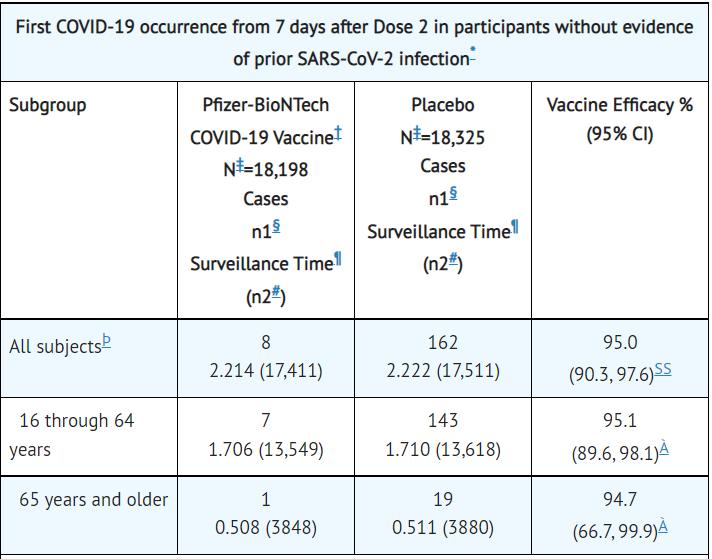
Clinical results
Vaccine components
Ingredient | mRNA | ALC-0315 | ALC-0159 | DSPC | Cholesterol |
Molar ratio (%) | - | 46 | 1.6 | 9 | 43 |
Other excipients: KH2PO4, Na2HOP4, potassium chloride, sodium chloride, sucrose, and water for injection | |||||
Market situation
Pfizer made $154 million in sales from its vaccine in 2020. Since 2021, vaccine sales has increased quarterly, which is closely related to the continuous strengthening of COVID-19 prevention and control and the promotion of vaccination in various countries. The sales of COVID-19 vaccine BNT162b2 boosted Pfizer earnings well past expectations in the third quarter of 2021, with nearly $13 billion. Pfizer sold $36.78 billion in vaccines in 2021. Vaccines are projected to bring $29 billion for Pfizer in 2022.
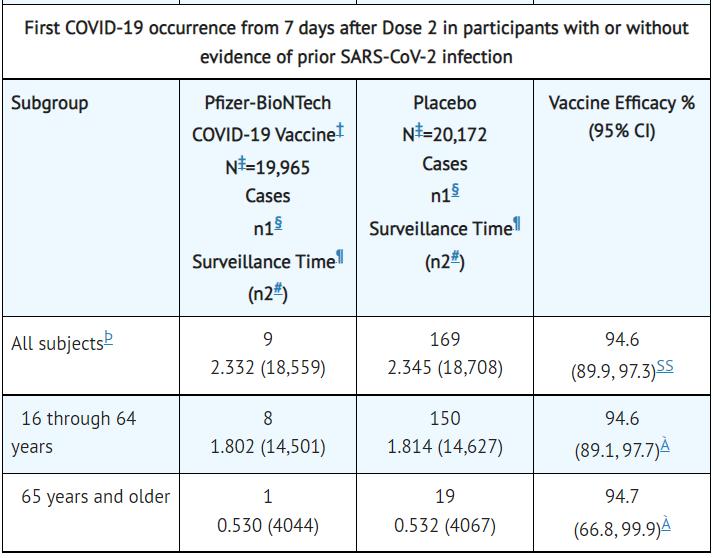
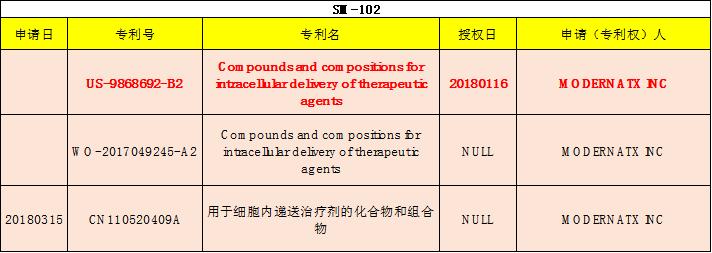
Clinical results
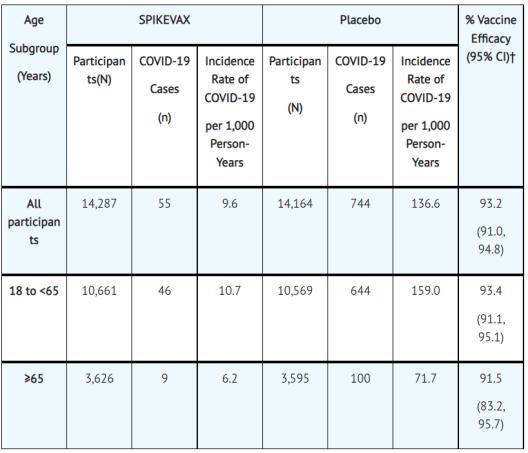
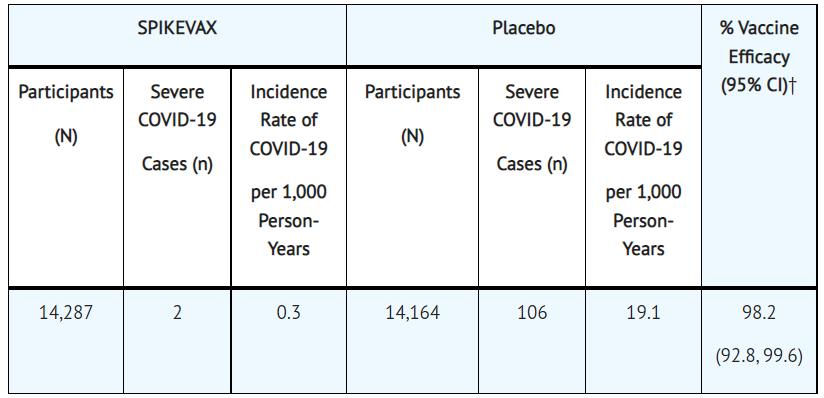
Vaccine components
Ingredient | mRNA | SM-102 | DMG-PEG2000 | DSPC | Cholesterol |
Molar ratio (%) | - | 50 | 1.5 | 10 | 38.5 |
Other excipients: Tris, sodium acetate, acetic acid, sucrose, and water for injection | |||||
Market situation
The full-year vaccine revenue for 2020 was $199 million, and the total revenue was $4.197 billion in the second quarter of 2021. Due to production and transportation problems, the vaccine revenue was $4.81 billion in the third quarter, at a significantly slowing growth rate. The COVID-19 vaccine revenue was $18.5 billion in 2021, which was less than the previous expectation of $20 billion. Based on the signed orders accounting to $17 billion, and the revenue for 2022 was predicted to be $17 billion to 22 billion.
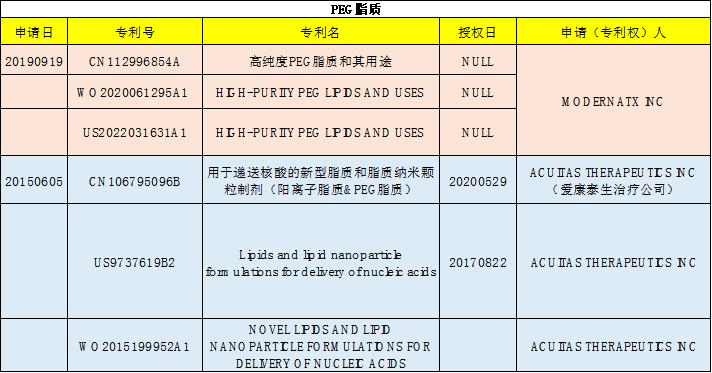
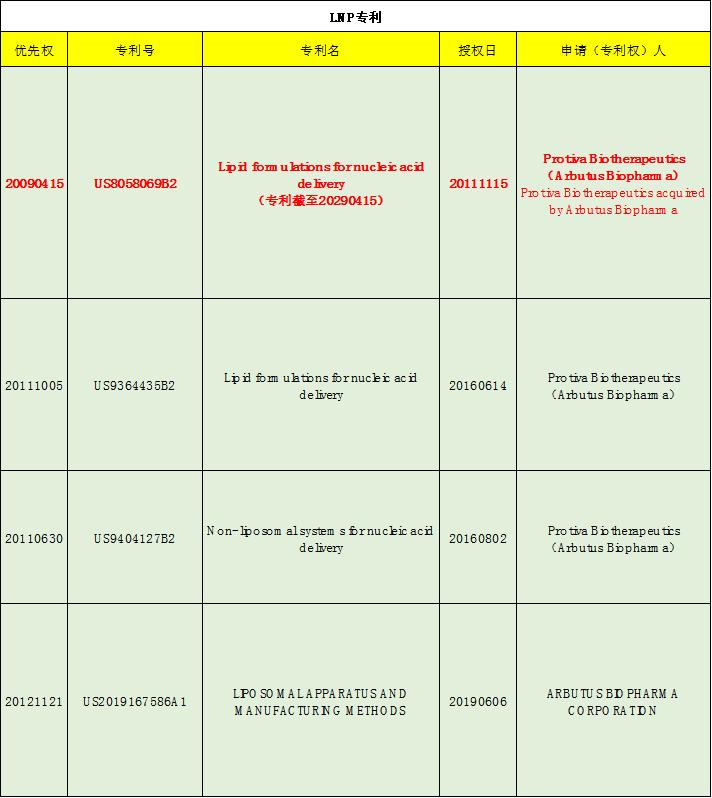
Our products
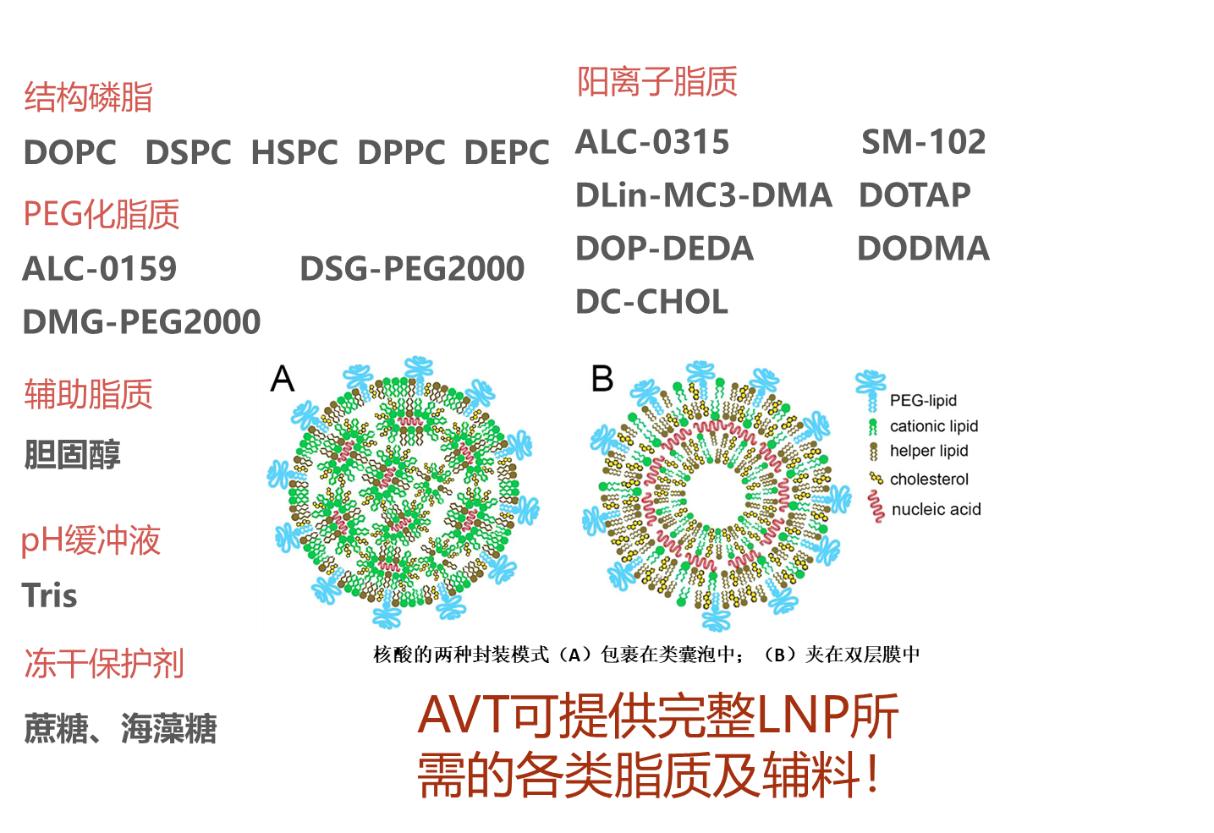
AVT supplies high-quality products such as cationic lipids, PEGylated lipids, phospholipids, cholesterol hp, Tris buffer, sucrose, and trehalose required for LNP. Moreover, we have been authorized for refinement of our Ionizable cationic lipid material DOP-DEDA. Contact us for more information.
AVT Ltd will work with you on the long road to RNA delivery for more product details, contact AVT at 400-6262-623.
Data and data are cited from:
1. Formulation information-DailyMed (nih.gov)
2. Patent information-EPO - Home
3. Clinical information-Home - ClinicalTrials.gov
4. Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021; 6(12):1078-1094. doi:10.1038/s41578-021-00358-0