In the first three issues, we shared the studies of D(+)-trehalose dihydrate in the cryoprotection of human oligodendrocyte precursor cells (OPCs), adipose-derived stem cells (ADSCs), mouse testicular germ cells, etc. These studies found that adding a suitable amount of D(+)-trehalose dihydrate to the cryopreservation medium or using a cryoprotectant (CPA) containing D(+)-trehalose dihydrate and glycerol can improve the recovery rate of cells after long-term cryopreservation.
In this issue, we develop a clear understanding of the cryoprotective effect of D(+)-trehalose dihydrate in three different human pluripotent stem cell lines through an article published in the Stem Cell Research in 2018 by researchers from Milan, Italy.
Cryopreservation of Human Pluripotent Stem Cells with D(+)-Trehalose Dihydrate
Cell therapy products, including stem cells, have high clinical potential. The global stem cell market involves upstream and midstream stem cell storage and preparation as well as downstream clinical applications. According to the report of Transparency Market Research (TMR), the global stem cell market is expected to reach US$ 270.5 billion by the end of 2025, and the compound annual growth rate of the market is on track to reach 13.8% in the past 8 years.
To ensure the successful application of cell therapy products, long-term cryopreservation of cells is a technical difficulty that must be conquered because cryopreservation is the only available method for long-term preservation of viability and biochemical function of cells.
In order to avoid damage to cellular structures caused by intracellular freezing, cryoprotectants are developed and marketed. The most commonly used cell cryoprotectants are dimethyl sulfoxide (DMSO) in combination with fetal bovine serum (FBS) or any serum substitute.
However, FBS contains xenogenic animal-derived proteins that may cause zoonotic infections with unknown pathogens. In addition, DMSO is cytotoxic. It is reported that the toxicity of DMSO on tissues and cells is directly related to exposure time, temperature, and concentration. The degree of toxicity varies according to cell type, and adverse effects have been reported in patients reinjected with thawed cells without removing DMSO. In addition, DMSO is known to affect the epigenome of mouse embryoids by regulating transcript levels of three DNA methyltransferases (Dnmts) and altering genome-wide methylation profiles, which in turn leads to uncontrolled differentiation of mouse stem cells. For all of these reasons, DMSO + FBS is not suitable for clinical use. There is an urgent need to develop efficient and non-toxic cryoprotectants.
D(+)-trehalose dihydrate is a non-reducing disaccharide found in a variety of organisms capable of surviving complete dehydration, such as bacteria, yeasts, tardigrades, and nematodes. Mammals do not produce D(+)-trehalose dihydrate, but it is an effective cryoprotectant of mammalian cells, which can reduce cell damage caused by ice crystal formation. The protective effect of D(+)-trehalose dihydrate is related to the osmotic effect and the specific interaction of cell membrane phospholipids and labile proteins, which can prevent cell damage and denaturation due to desiccation and oxidative stress.
Non-cytotoxic D(+)-trehalose dihydrate has been effectively used for cryopreservation of different types of cells such as mouse spermatocytes, adult hematopoietic stem cells, mesenchymal stem cells, adipose-derived stem cells (ADSCs), human embryonic stem cells (hESCs) and human-induced pluripotent stem cells (hiPSCs). D(+)-trehalose dihydrate is also used for cryopreservation of cord blood, bone marrow, and human islets.
The main obstacle preventing the widespread use of D(+)-trehalose dihydrate in cell preservation is that it is difficult for D(+)-trehalose dihydrate to access the interior of the cell. Several means have been applied previously to address the problem, such as osmotic shock, liposome delivery, heat perforation, electroporation, microinjection, and genetic engineering. However, the above methods require complex operations, are laborious and time-consuming, and may lead to significant cell damage.
In this study, three different types of pluripotent stem cell lines were cryopreserved with four different cryoprotectants prepared using D(+)-trehalose dihydrate alone or in combination with ethylene glycol or glycerol, respectively. The cell lines were then thawed to investigate the key parameters including cell morphology, post-thaw viability, pluripotency marker expression levels, genomic stability, endoplasmic reticulum (ER) homeostasis, and DNA damage response, measuring the cryoprotective capacity of the four cryoprotectants on pluripotent stem cells.
Different Cryoprotectant Compositions
Group | Component |
A | 0.5 M D(+)-trehalose dihydrate |
B | 0.5 M D(+)-trehalose dihydrate + 2.5% ethylene glycol |
C | 0.5 M D(+)-trehalose dihydrate + 10% ethylene glycol |
D | 0.5 M D(+)-trehalose dihydrate + 10% glycerol |
All cryoprotectants were freshly prepared and diluted in phosphate buffered saline (PBS). Cells used in the study were cryopreserved using CS10. CS10 containing 10% DMSO is a serum- and animal component-free cryoprotectant, which is recommended for cryoprotection of human pluripotent stem cells (hPSCs).
Culture the cells under specific conditions. Dissociate hESCs-RC17 with 0.5 mM EDTA, treat hiPSCs-CTR2#6 and ltNES-AF22 with cell detachment solutions, and store in freshly prepared cryoprotectants (A, B, C, and D). Resuspend 2.0 × 10^6 cells in 1.5 mL of each cryoprotectant and transfer to cryogenic vials.
Cool cryogenic vials in a freezing container at approximately -1℃/min, and transfer to a freezer at -80℃ after overnight storage. After 24 h, transfer cryogenic vials to liquid nitrogen for storage for at least one week.
Warm the cryogenic vials in a water bath at 37℃ until the ice mass disappears, and dilute the cell suspension with warm medium. Collect cells of 200 g of tissue by centrifugation for 3 min and seed onto coated culture vessels appropriate for each cell type at the indicated inoculum size.
① Cell viability
Measure the cell viability with the agent alamarBlue 48 h after thawing.
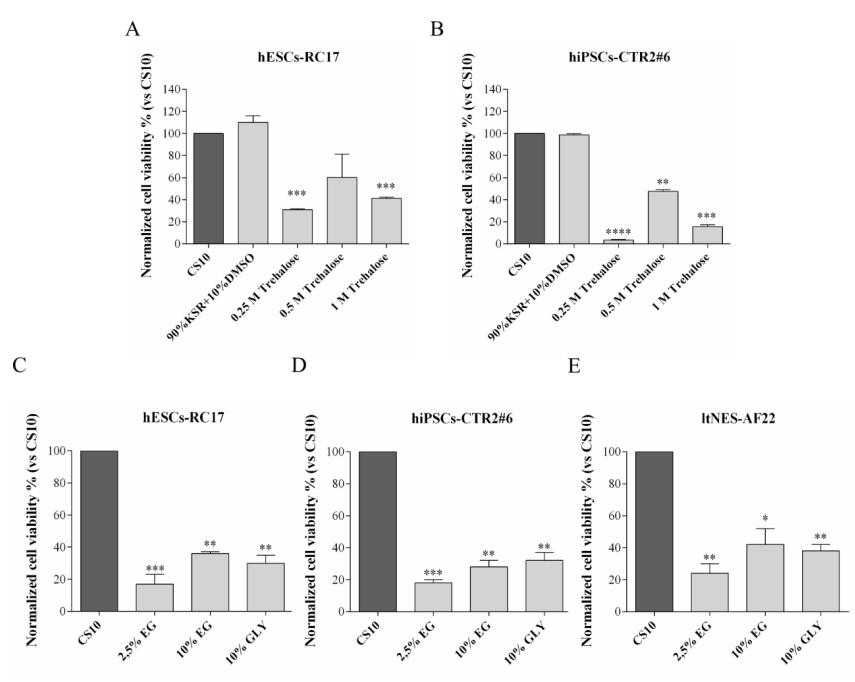
First, to determine the appropriate amount of D(+)-trehalose dihydrate, ethylene glycol, and glycerol, different concentrations of single component solutions were used for different stem cell lines. The viability of the cells after thawing was measured and compared with control groups. The results showed that 0.5 M D(+)-trehalose dihydrate, 10% ethylene glycol and 10% glycerol were appropriate amounts, but there were great differences among different stem cell types.
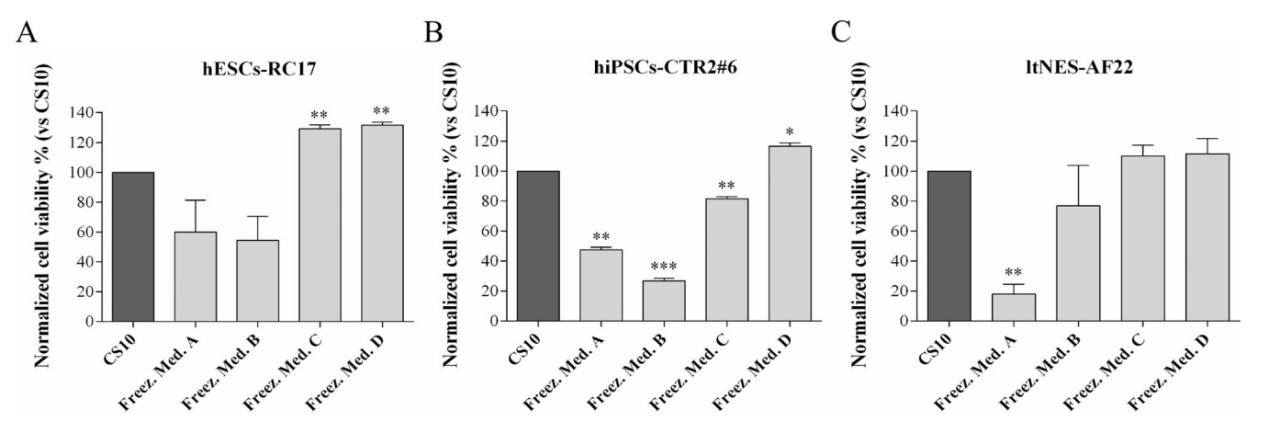
After determining the initial concentration, four different cryoprotectants (see above) were used for cryopreservation of three stem cell lines: (Group A) hESCs-RC17, (Group B) hiPSCs-CTR2#6, and (Group C) ltNES-AF22. The viability of the cells was measured after thawing. The cell survival rate was also compared to the same cells cryopreserved in CS10 under the same conditions.
When cells were cryopreserved with D(+)-trehalose dihydrate only (Group A), the recovery rate of cells after thawing decreased, varying from 20% to 60% for different cell types.
When D(+)-trehalose dihydrate-based medium was supplemented with 10% ethylene glycol or 10% glycerol (Groups C and D), the cell survival rates of hESCs-RC17 and ltNES-AF22 reached similar levels as those cryopreserved in DMSO. However, the cell survival rate of hiPSCs-CTR2#6 cryopreserved in glycerol was better than that in ethylene glycol.
In addition, comparing the results with control cryoprotectants containing ethylene glycol or glycerol alone, it was confirmed that the increased survival rate was due to the combined effect of ethylene glycol/glycerol + D(+)-trehalose dihydrate.
These results suggest that D(+)-trehalose dihydrate can be used for cryopreservation of human pluripotent stem cells and is expected to replace DMSO, and adding 10% ethylene glycol or 10% glycerol can significantly improve the survival rate of trehalose cryoprotectants. The mean cell survival rate was increased compared to CS10. Cryoprotectants of ethylene glycol/glycerol and D(+)-trehalose dihydrate do not contain serum proteins and avoid the previously reported risk of stimulating premature differentiation of pluripotent stem cells.
② Cell morphology
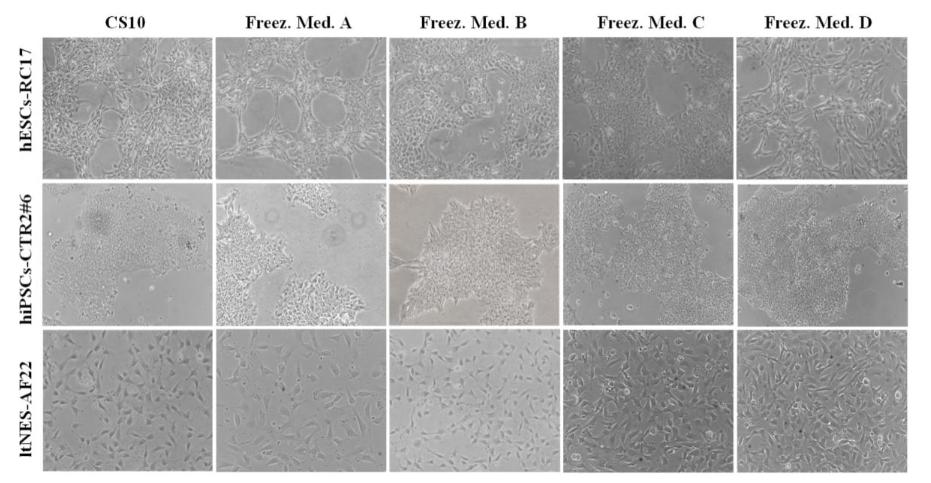
Another concern with stem cell cryopreservation is chromosomal and pluripotency alterations. During freezing and thawing, ice crystals and bubbles may form inside and outside the cells, disrupting spindle microtubules to induce abnormal chromosome segregation and resulting in changes in cell growth characteristics and morphology. Therefore, the possibility of damage to the chromosome and pluripotent potential of stem cells can be easily assessed by observing the morphology of the cells after thawing.
The upper figure shows the cell morphology of hESCs-RC17, hiPSCs-CTR2#6 and ltNES-AF22 48 h after thawing. Phase contrast images showed that composite D(+)-trehalose dihydrate cryoprotectants did not cause significant morphological changes in any of the cell lines, and the cell morphology was identical to that of cells preserved in CS10.
③ Marker expression level
To confirm that hESCs-RC17, hiPSCs-CTR2#6, and ltNES-AF22 after thawing retained the key properties and characteristics of pluripotent stem cells, the expression levels of several markers were analyzed by quantitative RT-PCR 48 h after thawing.
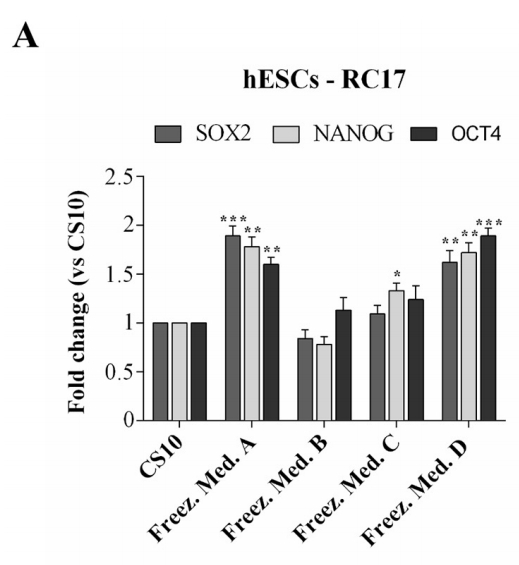
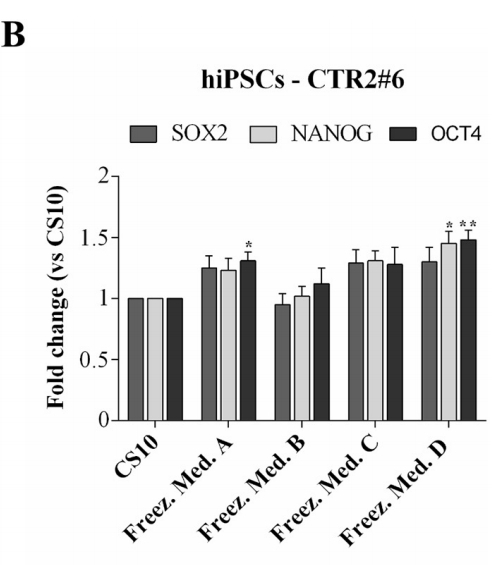
Cell markers Nanog, Oct4 and Sox2 were detected for hESCs-RC17 and hiPSCs-CTR2#6, and compared with control groups.
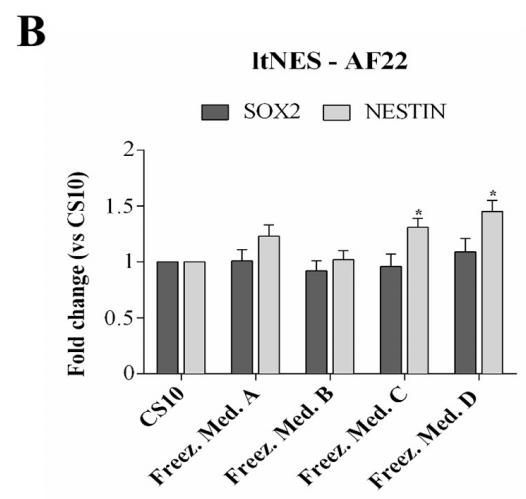
Nestin and Sox2 were detected for ltNES-AF22 and compared with control groups. The results showed that stem cell differentiation potential was not affected.
④ Chromosome stability
To assess the effect of D(+)-trehalose dihydrate-based cryoprotectants on the chromosome stability in stem cells, conventional karyotyping and aCGH were performed on all three stem cell lines. The figure below shows the results of ltNES-AF22 before and after cryopreservation.
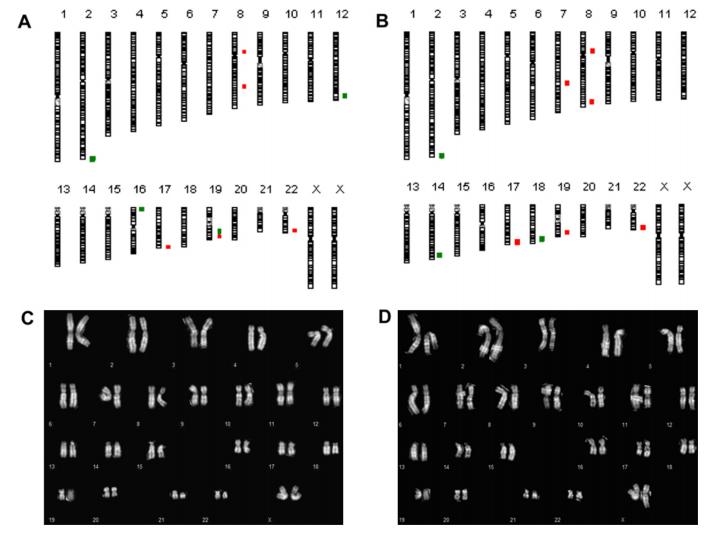
Overall, the karyotypes remained stable, but copy number variations (CNVs) were detected (chromosomes 2, 8, 19, and 22). Some of CNVs may have been present in the primary cells and others may have arisen randomly during in vitro manipulation of the cells rather than cryopreservation, as no clonal aneuploidy or structural chromosome aberrations were observed before (figure C) and after (figure D) cryopreservation.
⑤ ER stress/unfolded protein level assessment
The study also focused on whether cryopreservation in CS10 or D(+)-trehalose dihydrate affected pluripotent cells' ability to respond to the endoplasmic reticulum (ER) stress and activate the unfolded protein response (UPR). To maintain homeostasis in different physiological or pathological conditions, ER integrates various molecular and cellular signals. Both ER stress and UPR mediate molecular and biochemical mechanisms that affect cell proliferation, differentiation and apoptosis.
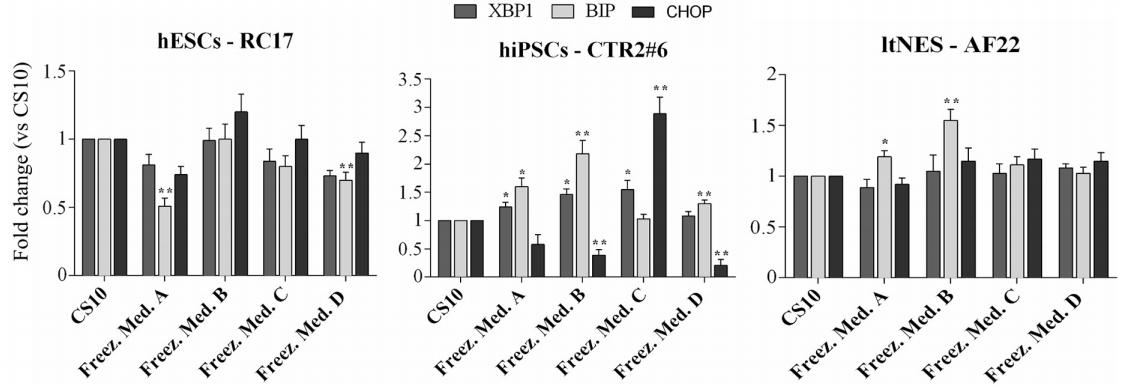
Overall, D(+)-trehalose dihydrate exhibited a moderate level of ER stress/UPR compared to CS10. The only more sensitive cell line was hiPSCs-CTR2#6, which showed higher BIP and CHOP gene expression levels in Groups B and C, indicating that cryopreservation with D(+)-trehalose dihydrate did not significantly alter the ER homeostasis of multipotent stem cells compared to CS10.
Long-term cryopreservation of stem cells has great application potential in many fields such as cell transplantation and cell therapy. Several studies have optimized the means for cryopreservation of human pluripotent stem cells, and long-term cryopreservation with D(+)-trehalose dihydrate may be an ideal method.
Human pluripotent stem cells preserved in D(+)-trehalose dihydrate cryoprotectants maintain cellular phenotypes and functional properties, indicating potential applications of D(+)-trehalose dihydrate in clinical therapy. Although further studies are needed to evaluate the biosafety of the cells cryopreserved with composite D(+)-trehalose dihydrate cryoprotectants, the combination of D(+)-trehalose dihydrate with ethylene glycol or glycerol, as a biosafety cryoprotectant with no cytotoxicity or animal-derived proteins, shows great promise for clinical applications.